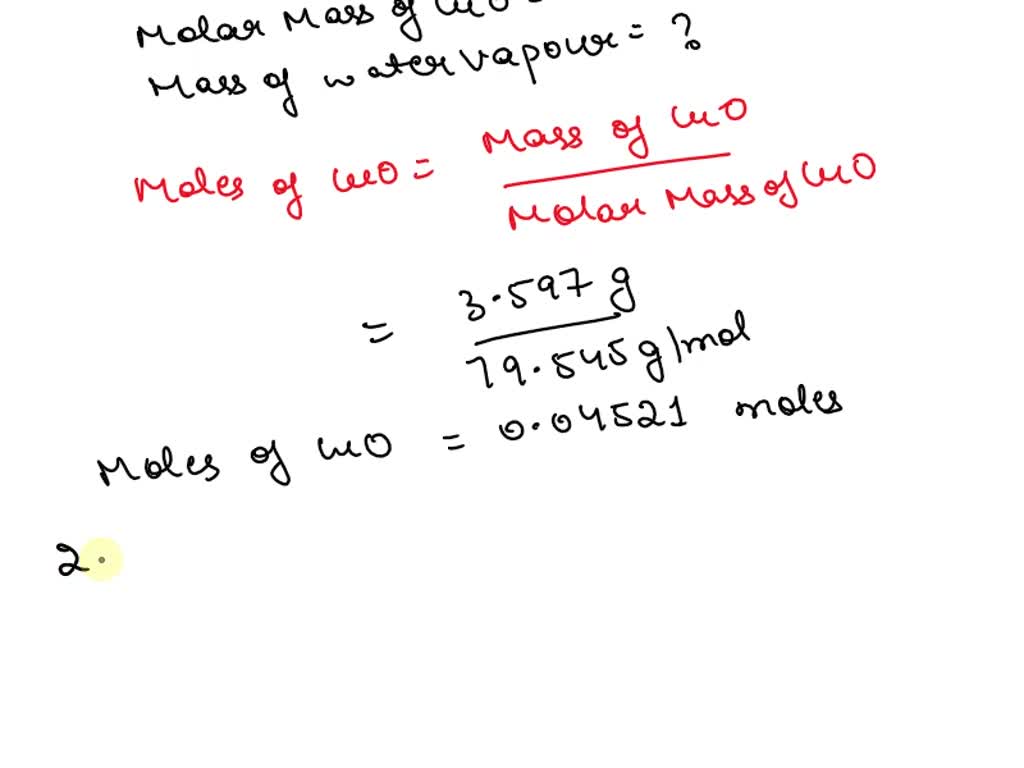Copper Ii Hydroxide Decomposition . in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. Cuoh = cu2o + h2o is a decomposition reaction where two moles. copper(i) hydroxide = copper(i) oxide + water. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. both synthesized materials were used as air desulfurization media at moist or dry conditions. Journal of physical chemistry c, 2019, 123 (34),. composition of copper(ii) hydroxide over different water vapor pressures. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium.
from www.numerade.com
Journal of physical chemistry c, 2019, 123 (34),. copper(i) hydroxide = copper(i) oxide + water. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. both synthesized materials were used as air desulfurization media at moist or dry conditions. Cuoh = cu2o + h2o is a decomposition reaction where two moles. composition of copper(ii) hydroxide over different water vapor pressures. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope.
SOLVED Experiment 2 Copper(II) carbonate hydroxide produces one mole of water (MM = 18.015 g
Copper Ii Hydroxide Decomposition both synthesized materials were used as air desulfurization media at moist or dry conditions. Cuoh = cu2o + h2o is a decomposition reaction where two moles. composition of copper(ii) hydroxide over different water vapor pressures. copper(i) hydroxide = copper(i) oxide + water. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. both synthesized materials were used as air desulfurization media at moist or dry conditions. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. Journal of physical chemistry c, 2019, 123 (34),.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Science online Copper Ii Hydroxide Decomposition Journal of physical chemistry c, 2019, 123 (34),. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Cuoh = cu2o + h2o is a decomposition reaction where two moles. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper.. Copper Ii Hydroxide Decomposition.
From www.youtube.com
[Mô phỏng thí nghiệm]💖Nhiệt phân Cu(OH)2🔥Thermal of Copper(II) Hydroxide📌Hóa học 9 Copper Ii Hydroxide Decomposition when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Journal of physical chemistry c, 2019, 123 (34),. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. Cuoh = cu2o + h2o is a decomposition reaction where two moles.. Copper Ii Hydroxide Decomposition.
From www.chegg.com
Solved Experiment 7 of Copper(II) Carbonate c. Copper Ii Hydroxide Decomposition when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. both synthesized materials were used as air desulfurization media at moist or dry conditions. composition of copper(ii) hydroxide over different water vapor pressures. Journal of physical chemistry c, 2019, 123 (34),. in this experiment, a weighed amount. Copper Ii Hydroxide Decomposition.
From www.youtube.com
Making Copper Oxide by Thermal YouTube Copper Ii Hydroxide Decomposition copper(i) hydroxide = copper(i) oxide + water. composition of copper(ii) hydroxide over different water vapor pressures. Cuoh = cu2o + h2o is a decomposition reaction where two moles. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. both synthesized materials were used as air desulfurization media. Copper Ii Hydroxide Decomposition.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Ii Hydroxide Decomposition Journal of physical chemistry c, 2019, 123 (34),. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. both synthesized materials were used as air desulfurization media at moist or dry conditions. copper(i) hydroxide = copper(i) oxide + water. composition of copper(ii) hydroxide over different water vapor. Copper Ii Hydroxide Decomposition.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation ID35476 Copper Ii Hydroxide Decomposition composition of copper(ii) hydroxide over different water vapor pressures. both synthesized materials were used as air desulfurization media at moist or dry conditions. copper(i) hydroxide = copper(i) oxide + water. Journal of physical chemistry c, 2019, 123 (34),. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. when carbon dioxide. Copper Ii Hydroxide Decomposition.
From www.chegg.com
Solved Experiment 2 Copper(II) carbonate hydroxide produces Copper Ii Hydroxide Decomposition copper(i) hydroxide = copper(i) oxide + water. composition of copper(ii) hydroxide over different water vapor pressures. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. both synthesized materials were used as air desulfurization media at moist or dry conditions. copper hydroxide was easily decomposed thermally. Copper Ii Hydroxide Decomposition.
From drqmlwefeco.blob.core.windows.net
Copper Ii Hydroxide And Ammonia at Lawrence Klein blog Copper Ii Hydroxide Decomposition in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. Journal of physical chemistry c, 2019, 123 (34),. both synthesized materials were used as air desulfurization media at moist or dry conditions. composition. Copper Ii Hydroxide Decomposition.
From www.numerade.com
SOLVED Texts Part III Preparation of Copper(II) Oxide From Copper(II) Hydroxide Cu(OH)2 → Copper Ii Hydroxide Decomposition composition of copper(ii) hydroxide over different water vapor pressures. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. copper(i) hydroxide = copper(i) oxide + water. both synthesized materials were used as. Copper Ii Hydroxide Decomposition.
From www.slideserve.com
PPT Types of Reactions Lab PowerPoint Presentation, free download ID2524104 Copper Ii Hydroxide Decomposition composition of copper(ii) hydroxide over different water vapor pressures. copper hydroxide was easily decomposed thermally or by electron irradiation, in an electronic microscope. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. Cuoh = cu2o + h2o is a decomposition reaction where two moles. Journal of physical. Copper Ii Hydroxide Decomposition.
From www.youtube.com
Making copper hydroxide YouTube Copper Ii Hydroxide Decomposition copper(i) hydroxide = copper(i) oxide + water. Cuoh = cu2o + h2o is a decomposition reaction where two moles. both synthesized materials were used as air desulfurization media at moist or dry conditions. composition of copper(ii) hydroxide over different water vapor pressures. Journal of physical chemistry c, 2019, 123 (34),. when carbon dioxide is dissolved in. Copper Ii Hydroxide Decomposition.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546 Copper Ii Hydroxide Decomposition in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Cuoh = cu2o + h2o is a decomposition reaction where two moles. Journal of physical chemistry c, 2019, 123 (34),.. Copper Ii Hydroxide Decomposition.
From www.numerade.com
SOLVED Write a balanced equation for the reaction that occurred in Experiment 2 Copper Ii Hydroxide Decomposition in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Cuoh = cu2o + h2o is a decomposition reaction where two moles. composition of copper(ii) hydroxide over different water. Copper Ii Hydroxide Decomposition.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the reaction of copper metal with Copper Ii Hydroxide Decomposition Journal of physical chemistry c, 2019, 123 (34),. composition of copper(ii) hydroxide over different water vapor pressures. Cuoh = cu2o + h2o is a decomposition reaction where two moles. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. copper hydroxide was easily decomposed thermally or by electron. Copper Ii Hydroxide Decomposition.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free download ID2858272 Copper Ii Hydroxide Decomposition Journal of physical chemistry c, 2019, 123 (34),. in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. copper(i) hydroxide = copper(i) oxide + water. Cuoh = cu2o + h2o is a decomposition reaction where two moles. composition of copper(ii) hydroxide over different water vapor pressures. both. Copper Ii Hydroxide Decomposition.
From www.youtube.com
Thermal of Copper(II) Carbonate YouTube Copper Ii Hydroxide Decomposition Cuoh = cu2o + h2o is a decomposition reaction where two moles. both synthesized materials were used as air desulfurization media at moist or dry conditions. composition of copper(ii) hydroxide over different water vapor pressures. copper(i) hydroxide = copper(i) oxide + water. Journal of physical chemistry c, 2019, 123 (34),. copper hydroxide was easily decomposed thermally. Copper Ii Hydroxide Decomposition.
From www.numerade.com
SOLVED Experiment 2 Copper(II) carbonate hydroxide produces one mole of water (MM = 18.015 g Copper Ii Hydroxide Decomposition in this experiment, a weighed amount of copper metal is transformed, through a series of reactions, into other copper. copper(i) hydroxide = copper(i) oxide + water. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. both synthesized materials were used as air desulfurization media at moist. Copper Ii Hydroxide Decomposition.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Ii Hydroxide Decomposition copper(i) hydroxide = copper(i) oxide + water. Cuoh = cu2o + h2o is a decomposition reaction where two moles. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Journal of physical chemistry c, 2019, 123 (34),. in this experiment, a weighed amount of copper metal is transformed,. Copper Ii Hydroxide Decomposition.